In-silico trials are an efficient way of improving the clinical trials process and getting both drugs and devices to patients more quickly, but experts in this area have struggled to persuade companies, researchers and regulators of their validity until recently.
The COVID-19 pandemic has highlighted the need for quick and effective trials that speed up the current slow progress of drug development. In-silico trials, carried out on virtual populations using computer modelling, have been discussed in the medical research and development space for a while. Until recently researchers and clinicians have been reluctant to explore them, perhaps due to fears about how accurately such trials can really represent a human population.
“I think the real time point in-silico clinical trials started to gain a lot of traction was in 2018 when the FDA received the congressional requirements to promote in-silico clinical trials. That was certainly from an industry standpoint, probably a turning point,” François-Henri Boissel, CEO and co-founder of Novadicovery, or ‘Nova’, a French company that has focused on in-silico trial development since launching in 2010.
“The agency nowadays has about 200 experts in what they call Model-Informed Drug Development, or MIDD, working across medical devices and drug development.”
In-silico trials really started on medical devices in the early 2000’s and have more recently moved more towards drug trials, according to Boissel. However, their relevance to device development is still of key importance.
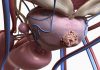
Last week, Alejandro Frangi, a professor at the Centre for Computational Imaging and Simulation Technologies in Biomedicine at the University of Leeds, and colleagues published a paper in Nature Communications demonstrating the value of such trials for medical device development.
They showed that an in-silico trial of intracranial flow diverters, designed to prevent brain aneurysm rupture, was able to replicate and expand on findings from conventional clinical trials of the same devices.
As well as demonstrating similar findings to the traditional clinical trials, the researchers also discovered new insights into why the devices do not work in some vulnerable patients, something they would not have been able to do in a standard trial.
“What in-silico trials try to do is to say, ‘Well, is there a way that we can reduce, replace, and refine testing mechanisms,” Frangi told Clinical Omics, emphasizing that these kinds of trials are not trying to replace conventional trials, but more to help them be more efficient and effective.
He explained that in-silico trials can be particularly useful for devices such as flow diverters, as patients with brain aneurysms are already high risk and so it’s important to minimize unnecessary clinical interventions.
“When we say ‘replace’ effectively what it means is we can do something we couldn’t do rather than we are not going to do what we usually do.”
These kinds of trials can also reduce the overall number of trials needed, according to Frangi. “Imagine you have 20 flow diverters and you could find out in-silico that there are 15 of that will never work because they have fundamental issues. You can already see this through simulations, so instead of doing 20 trials, you do five.”
Another important aspect of clinical trials is statistical power. This is crucial to get right when designing trials. “What you don’t want is what happens sometimes with current trials. Halfway through the process, they realize that they are not powered enough, and either they need to extend them or to stop them for financial reasons,” says Frangi.
In-silico trials can also help to refine this process. “Knowing a priori, the magnitude through simulations of what you roughly expect, allows you to have a sense of what is the right trial that you need to do.”
Both Frangi and Boissel emphasize the huge potential that in-silico trials have to bring down the overall cost of clinical trials and to speed up drug discovery and medical device approval.
“The current system for doing medical device innovation is completely broken…. We are still doing fundamentally the same process of medical device innovation that we did 30 years ago, which is fundamentally a glorified trial and error approach,” says Frangi.
“In the past, we used wind tunnels to test automotive automobiles. Now we use digital simulations to actually look at aerodynamics, but we also do virtual prototyping, and 3D printing to build prototypes that we can use to test aspects of ergonomics…. All of those technologies have made a massive difference in the automotive industry, what we want to do is see how we could utilize similar technologies in the medical device domain.”
One aspect that has held the field back, is persuading non-experts that doing these trials is a good idea and that it will provide accurate results.
Boissel and his colleagues at Nova are working hard to allay the fears of company’s and researchers interested in exploring the in-silico trials avenue.
“Confidence for non-subject matter experts is something that we built from the ground up in our clinical trial simulation platform, which is called Jinko. Each part of the system and the model is traceable back to its primary source, so that someone who does not know anything about mathematical equations and computer programming, can actually go back to the exact scientific articles that were used to build the textual and graphical version of the disease model before it was converted into mathematical equations.”
The company is also a founding member of the Belgium-based Avicenna Alliance, a non-profit association set up to improve awareness of in-silico trials and set up industry and research standards for regulating such trials.
“We’re working towards the drafting of good simulation practices, which obviously will include a framework for satisfactory validation of those disease models. What we’re trying essentially to achieve is to reproduce the results of existing experiments,” explained Boissel.
The pandemic has in some ways been beneficial for companies and researchers working in this area, as the value of in-silico trials was highlighted. “Clinical trials ground to a halt and pharma companies wanted to continue to explore some hypotheses on their drug programs and use in-silico methods,” said Boissel.
Despite this new interest, he reiterates that in-silico trials are not a direct replacement for traditional trials. “In the long run, I think the way in-silico is best used is on a systematic basis before more conventional experiments, whether you’re in preclinical or clinical development, so that you can test a large number of assumptions and identify the best scenarios that need to be tested.”